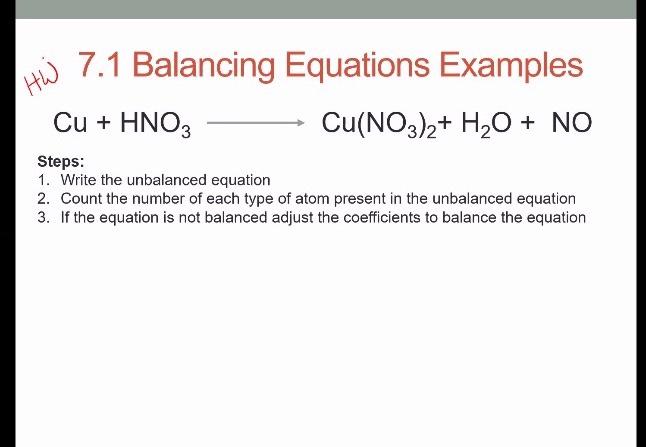
Balance the chemical equation: (1) Cu + HNO3 → Cu (NO3)2 + H2O + NO2 (2) NH3 + Cl2 → - Science - Chemical Reactions and Equations - 12597703 | Meritnation.com

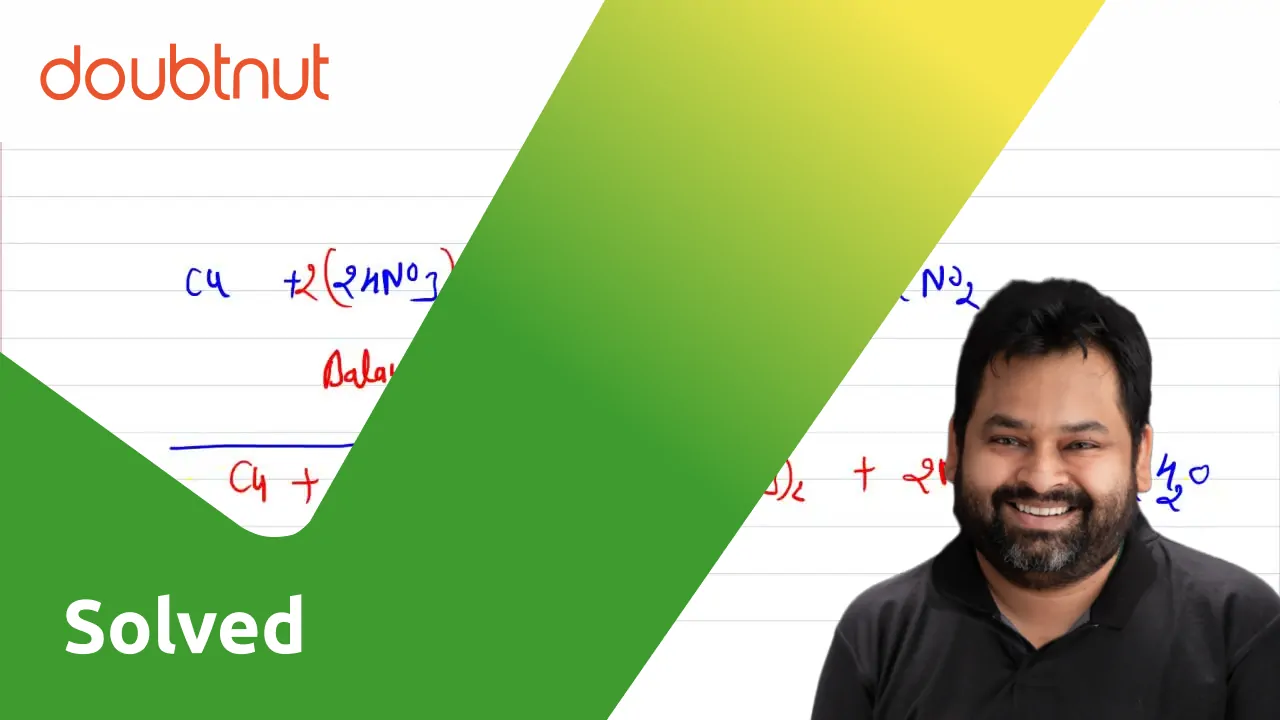
Algebraic method or a,b,c method for balance the equation. Cu+HNO3=Cu(NO3)2+NO2+H2O balance the equa - YouTube
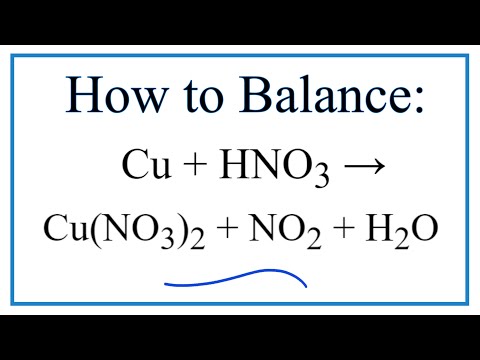
SOLVED: Balance the following equation : Cu(s) + HNO3(aq) → Cu(NO3)2 (aq) + NO(g) + H2O(l) Now add the coefficients. the sum of the coefficients is: a. 15 b. 18 c. 20 d. 17
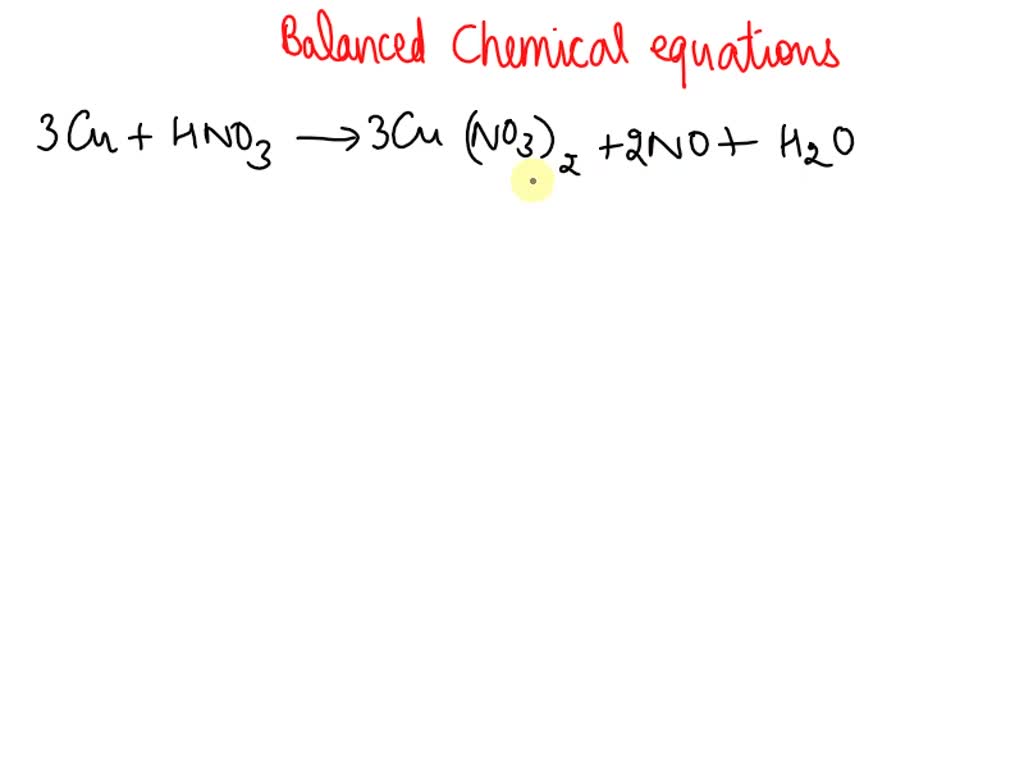
Complete and balance the following chemical reactions (i) Copper reacts with HNO3 to give NO and NO2 in molar ratio of 2 : 1 - Sarthaks eConnect | Largest Online Education Community
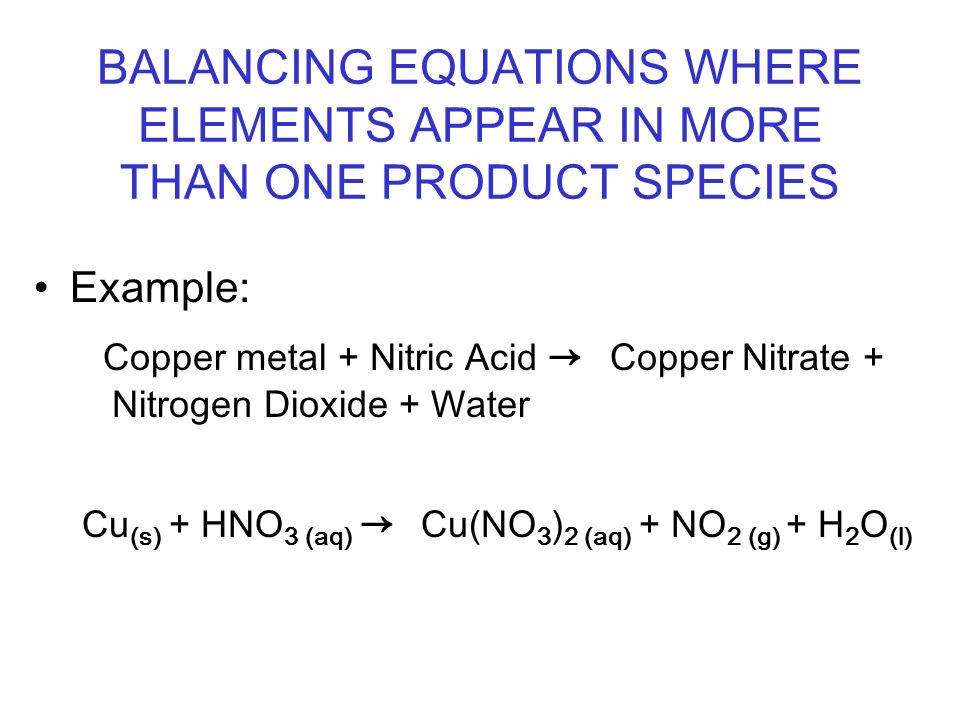
BALANCING EQUATIONS WHERE ELEMENTS APPEAR IN MORE THAN ONE PRODUCT SPECIES Example: Copper metal + Nitric Acid → Copper Nitrate + Nitrogen Dioxide + Water. - ppt video online download
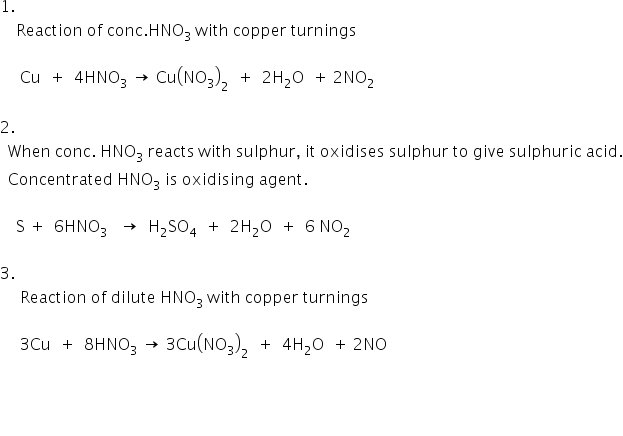
1) write a balanced equation for the reaction of conc.HNO3 when added to copper turnings kept in a beaker.2) write a balanced equation for the reaction of sulphur and hot concentrated nitric

Algebraic method or a,b,c method for balance the equation. Cu+HNO3=Cu(NO3)2+NO+H2O balance the equat - YouTube